Photo-uncaging means the activation of biochemically masked (’caged’) molecules via photolysis that mimics physiological release of bioactive compounds/ messengers. The method allows to capture transient signals which are intrinsic to signal transduction. Here, the authors describe how photo-caging can be applied to investigate lipid-mediated signaling pathways and showcase a new uncaging system that can specifically deliver caged lipids into the mitochondria.
As basic units of life, it is essential for cells to receive and correctly respond to signals. Typically, signaling molecules first activate specific receptors on the cell surface, which in turn trigger corresponding intracellular pathways. When the signals are transferred to the intracellular space, many small molecules take over and serve as faithful messengers to activate numerous downstream effectors. Not surprisingly, many lipids are important secondary messengers due to their abundance in cell membranes. Accordingly, aberrant lipid levels are often linked to a broad range of diseases such as cancer, diabetes, and neurodegenerative disorders.
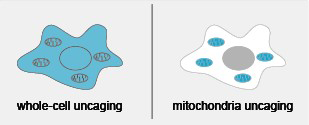
In order to better understand intracellular signaling networks, genetic knockout, RNA interference (RNAi) and small-molecule inhibitors are common ways of targeting key enzymes of the metabolic pathways. However, these techniques cannot capture transient signals which are intrinsic to signal transduction. In order to improve the temporal resolution, photo-uncaging has been introduced as an alternative solution. Instead of focusing on the enzymes, the uncaging technique works with the actual messenger molecules themselves. Starting with chemical modification, the messenger molecules are first protected by photolabile groups and hence lose their bioactivities. When applied to cells, the protecting groups can be quickly removed by a flash of light and thereby release the bioactive molecules. In this way, only corresponding downstream pathways are activated, and thus it offers a unique chance to study a specific signaling network. To date, photo-uncaging has been widely used for signaling studies especially for neurotransmitters. In the recent years, a number of caged lipid molecules have also been introduced to investigate lipid-mediated signaling pathways (1). While these methods greatly enriched the toolbox to explore lipid signaling, they are also limited by the random distribution of caged lipid precursors. Intriguingly, depending on the subcellular compartment, many lipid messengers could serve distinct functions in the cells. Recently, we developed a new uncaging system that can specifically deliver caged lipids into the mitochondria (2). Compared to previous techniques, this new mitochondria-targeted uncaging method (mito-cage) significantly improved the spatial resolution.
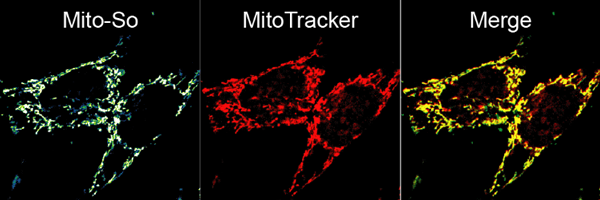
Coupling the coumarin molecule, a commonly used photolabile group, to a popular mitochondria targeting motif (TPP) allowed us to deliver caged sphingosine (names as Mito-So) into the mitochondrial matrix in intact cells. Combining this method with highly sensitive mass spectrometry, for the first time we were able to monitor the mitochondrial sphingosine metabolism in real-time. Using an isotope-labelled lipid, we found that whole-cell uncaged sphingosine quickly converts into ceramide, while the conversion of sphingosine to ceramide is much slower after mitochondria-specific uncaging. Furthermore, sphingosine released globally in the cell was able to mobilize calcium whereas even higher amounts released inside the mitochondria did not. These results demonstrate the importance of subcellular localization on lipid metabolism and signaling. In the future, caged molecules targeted to many other organelles will certainly help to draw a clearer picture of complex signaling networks and reveal novel information about the spatial regulation of lipid metabolism.
References
- Doris Höglinger; André Nadler; and Carsten Schultz, Caged lipids as tools for investigating cellular signaling, Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2014.
- Feng, Suihan, et al., Mitochondria-specific photoactivation to monitor local sphingosine metabolism and function, eLife 7, 2018.

Leave a comment
The editors reserve the right not to publish comments or to abridge them.