Five research teams of the NCCR Chemical Biology join efforts to use bioorthogonal chemistry answers to develop novel chemistries to specifically modify or influence biological processes, as well as to deliver novel molecules to expand the chemical diversity of small molecules.
Interview with Professor Jérôme Waser, Principal Investigator
Could you give us some insight in the project and the approaches that are being pursued?
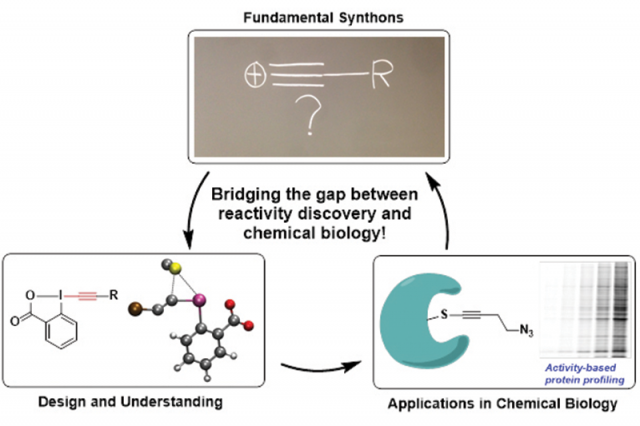
The concept of bioorthogonal chemistry and chemical diversity are, at the basis, different. Bioorthogonal reactions normally involve functional groups not existing in nature, which can react without interference from the biomolecules. The most well-known example is the [3+2] cycloaddition of alkynes and azides (“Click chemistry”). On the other hand, efforts toward chemical diversity seek to discover innovative molecular scaffolds which can interact with biomolecules. In principle, bioorthogonal reactions can also be used to increase chemical diversity by linking biomolecules with small organic compounds, such as natural product derivatives. In our laboratory, we indeed followed both goals. We first developed new annulation reactions to access nucleoside analogues with high chemical diversity. More recently, we changed our focus to the use of hypervalent iodine reagents for biomolecules functionalization. Hypervalent iodine compounds are not found in biological systems and combine high reactivity with stability in presence of water and most functional groups present in biomolecules. We therefore consider that they have an important potential to make a strong impact into the field of biomolecule functionalization.
What do you see as the major achievements of this project over the past 6 years, and for the last year in particular?
The first key breakthrough was in fundamental chemistry in 2012-2013. At that time, we discovered that the EthynylBenziodoXole (EBX) reagents used in our laboratory were reacting extremely fast with thiol anions to give the corresponding thioalkynes. This transformation was finished in a few seconds and was selective for sulfur nucleophiles in presence of many other reactive functional groups, such as amines, alcohols or electron-rich aromatic rings. We were also able to elucidate an unprecedented concerted reaction mechanism which rationalized the high rate of the reaction.
Whereas this first breakthrough was done exclusively in our laboratory (LCSO), the NCCR Chemical Biology network was truly instrumental to bring this project to the interface between biology and chemistry. We were indeed very interested in testing the method for the functionalization of cysteines in proteins, but were lacking the expertise for working with biomolecules. In this respect, an important proof of concept was first realized by the van der Goot group, which demonstrated that EBX reagents were indeed highly efficient to block cysteines in proteins. However, using EBX reagents only as “protecting group” was a little bit disappointing. The first truly exciting application was then developed by the Adibekian group in 2015, who used one of the synthesized reagents (JW-RF-010) for the proteome-wide profiling of targets of cysteine-reactive small molecules. This reagent, which bears also a reactive azide group for easy further functionalization, was superior both in selectivity and efficiency to state-of-the art iodoacetamide-based reagents. It allowed the identification of a unique set of cysteine-reactive proteins. One of them, casein kinase I gamma could be subsequently identified as a target of the natural product curcumin, which could help to better understand the anti-cancer activity of this compound as this protein is phosphorylating Akt, a known oncogene.
With the proof of concept established for the use of EBX reagents for biomolecule functionalization, this last year has been focused on new exciting directions. Our laboratory has continued to work on new reagents and the understanding of the reaction in collaboration with the Fierz group. Water soluble reagents are now available. Together with the Adibekian group, we are now also developing EBX reagents as selective inhibitors of proteins instead of broadly reacting reagents. Applications are by no means only limited to the functionalization of biomolecules. Indeed, any transformation involving the functionalization of sulfur atoms can profit from the amazing reactivity of EBX reagents. For example, the Matile group recently used EBX reagents as terminators for the polymerization of cell-penetrating polysulfides.
How was the process of the conception and development of the hypervalent iodine reagents? Did it start out from an idea or was it an accidental finding? What were the roadblocks and how did you deal with these?
As often in science, a mix of luck and design led to success. The project started purely on paper, with a fundamental challenge: can we transform an alkyne, an inherently nucleophile entity, into a electrophile? This was first a fundamental question of reactivity, which are those my group is most fascinated with. However, it was also a concept with broad potential for applications, as alkynes are frequently used in synthetic chemistry, materials science and chemical biology. Before our work, only less stable alkynyliodonium salts have been used for this purpose, but we were not able to develop useful new transformations with these very sensitive compounds. It is then that we first used more stable cyclic reagents. After that, Dr. Reto Frei had the idea to use it to functionalize thiols, and it worked like a charm. Kick-off for the biomolecule functionalization was a presentation by Matthieu Blanc from the van der Goot group working on palmitoylation during an NCCR meeting, after which we just decided to try it! Luck, unexpected opportunities, design and surprises continue since then to bring the project forward.
What do you see as the prime advantage of these reagents?
The unique combination of high reactivity with good stability, and their extreme “love” for sulfur, which make them ideally suited for the functionalization of biomolecules.
Where do you see the major applications of these reagents, and where could they have the biggest impact? In synthesis, biomolecular labelling or proteomics?
It is difficult to draw limits to what can be done with these reagents! Synthetic applications continue to be heavily investigated in our laboratory and around the world, with more than 60 applications on small molecules reported. Biomolecule labelling, in particular in living cells, has just begun to be investigated, and for that the reagent JW-RF-010 appears ideally suited and has a high potential for proteomics application. Biomolecular labelling on purified peptides and proteins begun to work also efficiently now, and the next goal would be to synthesize “smart” reagents containing fluorophores, cross-linkers, photo-activating groups and many more exciting possibilities. Use of such reagents to build up antibody conjugates or link biomolecules together can also be envisaged.
What would be your next “dream” compound or reaction?
We want to push the dream in two extreme directions: develop on the one hand reagents with very high general reactivity which are able to functionalize extensively biomolecules and on the other hand highly selective reagents, which will target a single protein in the cell. Preliminary results obtained by the Adibekian group indeed indicate that such a dream may become reality one day.
 Jérôme Waser is an Assistant Professor at the EPFL where he is Head of the Laboratory of Catalysis and Organic Synthesis (LCSO).
Jérôme Waser is an Assistant Professor at the EPFL where he is Head of the Laboratory of Catalysis and Organic Synthesis (LCSO).

Leave a comment
The editors reserve the right not to publish comments or to abridge them.