Selective in vivo metabolic cell-labeling-mediated cancer targeting
From Nature Chemical Biology (February 2017)
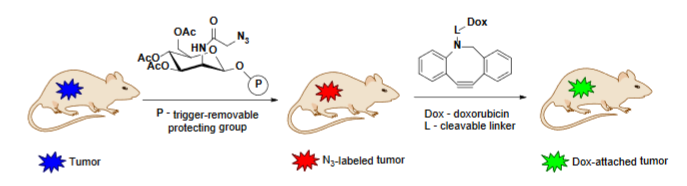
Selective targeting of cancer cells through surface chemical receptors is an approach for diagnostics and therapy of cancer. This paper by Hua Wang and co-workers demonstrates the method of selective labeling of cancer cells both in vitro and in vivo via designing the unnatural sugars that enable the selective introduction of azido-groups on the surface of tumor cells. The authors developed the tetraacetyl-N-azidoacetylmannosamine (Ac4ManAz) analog where the anomeric acetyl group was converted to a caged ether bond that can be selectively digested by tumor-overexpressed enzymes allowing the overexpression of azido groups on the cancer cells. Subsequent click-reaction of the cell surface azides with dibenzocyclooctyne-doxorubicin conjugate via click chemistry led to selective in vivo labeling of cancer cells and enhanced tumor accumulation of the antitumor drug doxorubicin. This technology, called ATTACK (active tissue targeting via anchored click chemistry), provided targeted cancer therapy against MDA-MB-231 breast cancer, LS174T colon cancer and 4T1 breast cancer in mice.
Assessing the mitochondrial membrane potential in cells and in vivo using targeted click chemistry and mass spectrometry
From Cell Metabolism (February 2016)
Understanding of mitochondria involvement in complex pathologies such as progeria, Alzheimer’s disease and diabetes was hampered by absence of a method to evaluate mitochondria function directly in living animals. This paper by Angela Logan and co-workers describes the development of such a method that is based on potential-dependent augmentation of the rate of a bioorthogonal reaction that takes place in the mitochondrial matrix. The authors incorporated a triphenylphosphonium moiety into a cyclooctyne derivative and an organic azide to make them accumulate in the mitochondrial matrix and facilitate detection of the reaction product by mass spectrometry. Using this approach, authors were able to detect depolarization of mitochondria in vivo by dinitrophenol and elevation of mitochondrial membrane potential in a mouse model of heart mitochondrial dysfunction.
Green- to far-red-emitting fluorogenic tetrazine probes-synthetic access and no-wash protein imaging inside living cells
From Chemical Science (February 2017)
Fluorogenic probes activated by bioorthogonal chemical reactions offer an advantageous approach in fluorescence microscopy applications to reduce signal background. This article by Richard Wombacher and coworkers describes the development and synthesis of different tetrazine-based fluorogenic dyes covering emission wavelengths from green to far-red. The tetrazine probes react with trans-cyclooctene via electron demand Diels-Alder cycloaddition, resulting in an enhancement in fluorescence emission. These newly developed probes were successfully applied in fluorescence microscopy imaging of intracellular proteins such as actin in fixed cells as well as nuclear and mitochondrial proteins in living cells. This report provides the first example of intracellular live cell protein imaging by the use of tetrazine-based fluorogenic probes without washing steps required.
Copyright main picture (Molecules): Designed by Freepik

Leave a comment
The editors reserve the right not to publish comments or to abridge them.