Endosomes are intracellular organelles which form part of the cell’s sorting center. The tension of cell membranes plays an important role in a number of biological processes. Scientists from the NCCR Chemical Biology have recently shown that the activity of endosomes is modulated by variations in membrane tension. Using molecular probes that they devised, the multidisciplinary team from the University of Geneva succeeded to evaluate the membrane tension of endosomes and show that its relaxation helps form vesicles within endosomes, which carry proteins to be degraded. We have met with Vincent Mercier, Post-doctoral researcher in the Roux lab, and first author of the relevant research paper, published in Nature Cell Biology.
Since your paper focuses on intracellular membrane tension, can you give us an introduction to what membrane tension is and why it is important to study it?
Membrane tension is a force that opposes surface area expansion. So, membrane tension will strongly depend, for example in the case of a compartment, on the volume of that compartment. It’s a little bit like a balloon: if you increase the pressure inside, you will increase the volume and consequently the tension of the membrane at the interface between two environments. If you decrease the volume or the pressure, you will decrease the tension. This is important because from that we can imagine that membrane tension will rapidly set in motion the deformability of the membrane; it will be harder to deform a membrane with higher tension. In addition to membrane tension, another important parameter for membrane deformability is the bending rigidity: if the rigidity is high, it will be hard to deform the membrane, but as this parameter will depend mainly on the composition of the membrane, bending rigidity’s variations will be at a longer timescale than membrane tension’s variation.
Why did you decide to study the role of membrane tension? In other words, what is the major focus of your study?
In this study, we focus only on endosomal membranes. Other research groups are already interested in this parameter, but as endosomes are intracellular, they are limited technically. Endosomes are not easily accessible with classical tools like tube-pulling or pipette aspiration, which are normally used to measure tension. The situation is different for us. With the new flipper-TR probe developed by the Matile group at the University of Geneva, we now have the opportunity to investigate this phenomenon.
What motivated the study? Did you observe something specific that made you decide to study endosomal response to membrane tension?
I joined the Roux lab at the University of Geneva to study the Endosomal-Sorting Complex Required for Transport (ESCRT) machinery which is involved in vesicle formation within endosomes. There were two key aspects that motivated this specific project and encouraged us to pursue it. Firstly, a previous study conducted by Matthieu Piel at the Curie Institute (Paris) suggested the existence of a link between the tension of the membrane and the action of the ESCRT machinery. Secondly, a former PhD student in our lab, Guillaume Molinard, observed a relocalization of the ESCRT machinery when treating cells with hypertonic solution, which decreases both plasma and endosomal membrane tension. Based on the latter evidence, we postulated that lower tension may promote the polymerization of ESCRT proteins. Finally, as the membrane is the substrate for the polymerization of ESCRT machinery – you need the membrane to have the polymerization – we thought that by increasing the deformability, i.e. by decreasing the tension, we would favour the polymerization of these proteins.
How did you modify endosomal membrane tension?
We applied hypertonic shocks because it is an easy way to modulate the tension of the membrane. The plasma membrane delimits the cell and separates the interior from the exterior. If you put hypertonic medium outside, you release water from inside the cell, which decreases the volume of the cell, and in this way you decrease the tension of the membrane. This is also true for endosomes inside the cell. By applying a hypertonic shock, you quickly decrease tension while with a hypotonic shock, such as by using water, you do the contrary, you rapidly increase membrane tension. The use of osmotic shocks therefore represents an easy tool to modulate membrane tension and investigate the effect on the ESCRT machinery.
And then, did you measure changes in membrane tension with the Flipper-TR probe?
Mainly yes, because you cannot have access to the tension of endosomes and measure it using current techniques like optical tweezers. We therefore took advantage of the probes developed by the Stefan Matile group. The Flipper-TR probe contains two DTT groups, which are flexible and can rotate; they can be in a planar or non-planar configuration. The probe inserts itself into the membrane between phospholipids and when you change the order of the lipids, you change the planarization of the probe – it can be twisted or non-twisted. Depending on the twisting and planarization, the fluorescence will change. What we measured is the lifetime of this fluorescence, which is basically the time during which the probe is emitting photons after an excitation pulse. Importantly, we know that by increasing the tension, you increase the formation of ordered domains of lipids, which will finally change the lifetime of the probe. Normally, fluorescence lifetime of the probe increases when you increase the tension in cells and lifetime decreases when you decrease the tension.
We were also fortunate to have a specific probe to label endosomes, the lyso Flipper-TR, also developed by the Matile group. This probe is basically the same as the one used for plasma membrane, but with a morpholine that has been added on it, which has basic pKa around 8.4. This allows the probe to specifically target acidic lysosomes and late endosomes and insert into their membrane. This way, we could precisely measure the lifetime of the probe in late endosomes and to see if there are differences before and after osmotic shocks. Other versions of the probe are available targeting the ER (endoplasmic reticulum) and the mitochondria, for example.
Are there plans to develop new probes?
We are now developing a new probe to target early endosomes, because the lyso Flipper-TR is targeting late endosomes and lysosomes with really acidic pH, whereas early endosomes have higher pH.
The Matile group has also developed halo-tag flipper, with which you can target every compartment in the cell. You have to transfect cells with a protein expressing half of the halo-tag, then you put your Flipper-TR with the other half which will target the specific compartment that you want to visualize.
In your study, you used both in vitro and in vivo experiments. What knowledge did you gain from each case?
We saw that hypertonic shock decreases the tension of endosomes and triggers polymerization of ESCRTs onto endosomal membranes. However, this was only correlative as we were not able to properly show that it was only due to a decrease in membrane tension. For this reason, we moved to an in vitro minimal system: we purified the main component of ESCRT-III, CHMP4B, and repeated the experiment with synthetic vesicles (liposomes) to show that membrane tension was regulating the polymerization of this protein.
A complementary experiment that we used to also modulate membrane tension was micropipette aspiration on liposomes. With this aspiration technique and simultaneous membrane tension measurements with optical tweezers (which permit to have access to the absolute value of membrane tension), we were able to show that there was a direct correlation between the polymerization of the protein CHMP4B and the tension of the membrane.
In vivo, you could not use the pipette aspiration technique because you were looking at endosomes, which are inside the cell. Whereas in vitro you could properly show that the increase in ESCRT polymerization is only due to membrane tension decrease. Is that correct?
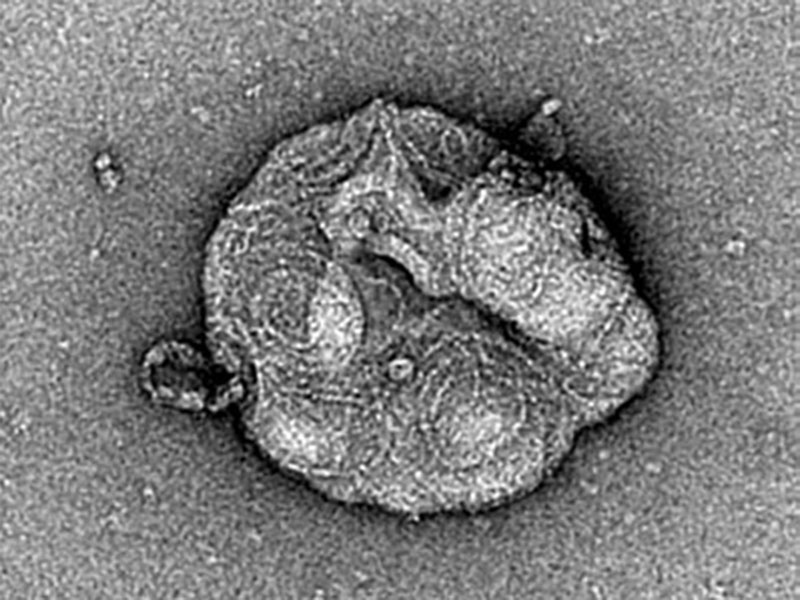
Yes, because in vivo we have the whole ESCRT machinery and not just the main component CHMP4B. One could argue that our results in vivo are due to an increase of the nucleators or proteins which are recruiting ESCRT-III but not to polymerization. Whereas in vitro, we can be sure that it is the polymerization since we only add the specific protein that polymerizes to form those filaments. This way, we were able to unambiguously show, using electron microscopy, that a polymerization process was taking place, and there was not just a random accumulation of protein on the membrane. The typical ESCRT spirals were really forming.
Why is your study important?
We know that membrane tension may vary really quickly and we thought that this ability could represent a very fast mechanism to transduce a signal of membrane deformation. In that direction, we observed that treating cells with EGF, which is a hormone triggering massive formation of endocytic vesicles but also intraluminal vesicles (ILVs) containing EGF receptor, decreases the tension of endosomal membrane. We hypothesized that the fusion of those endocytic vesicles with endosomes would decrease their membrane tension and promote the formation of ILVs by the ESCRT machinery, which then would restore normal membrane tension. We demonstrated in this study that it is the case and highlighted the fact that membrane tension can control organelle functions and cell physiology.
Could this be a mechanism to respond to signals that are not in the timescale of signalling pathways, but rather mechanical signals?
Yes, it is purely mechanics. You can also think that it may represent a way for the cell to save energy. The mechanics of the compartment (membrane tension) set a point of equilibrium for proteins activity, when for instance the volume of the compartment is changing it either facilitates or inhibits proteins polymerization.
What are your next steps? Are you continuing on this project or moving to something else?
I want to continue to study the role of membrane deformability on cellular processes involving membrane deformation. For instance, we know that there is a specific lipid enriched in late endosomes, called LBPA, so I’m wondering if LBPA will change the biophysical/mechanical properties of the membrane such as rigidity. I am now trying to measure the stiffness of liposomes with or without LBPA in vitro.
I would also like to investigate the role of membrane tension in neuronal synaptic activity because I think it is a system which requires this fast membrane deformation signal provided by variations of membrane tension. Indeed, during synaptic transmission, there is massive fusion of neurotransmitter vesicles with the limiting membrane of the pre-synaptic neuron. I think that this massive fusion will decrease plasma membrane tension and promote the biogenesis of new neurotransmitter vesicles. The balance between vesicle fusion and fission could be an elegant way to passively regulate membrane deformations through membrane tension in the presynaptic compartment.
How do you measure stiffness?
Using tube-pulling experiments and Giant Unilamellar Vesicles (GUVs), with or without LBPA at different concentrations. Then, using a micropipette, we hold the GUV and using a bead trapped in an optical tweezers, we pull a membrane nanotube. This way, we can measure the force exerted on the bead. Increasing aspiration of the GUV increases membrane tension. Finally, the relation between the force and the aspiration is used to measure the rigidity: if the rigidity is high, even a small increase of the aspiration will strongly increase the force.
Thank you Vincent, and congratulations for your beautiful paper!
—
Source:
Mercier, V., Larios, J., Molinard, G. et al., “Endosomal membrane tension regulates ESCRT-III-dependent intra-lumenal vesicle formation“, Nat Cell Biol 22, 947–959 (2020).

Leave a comment
The editors reserve the right not to publish comments or to abridge them.