Membrane is active both physically and biochemically, in influencing cell processes ranging from vesicle trafficking to actin assembly. A major challenge has been the development of reliable tools to image membrane tension. This article describes the course of innovation behind the birth of the fluorescent flipper probe.
The objective of the NCCR Chemical Biology is to visualize biological processes using chemistry. In this context, one of the most persistent challenges is the visualization of mechanical forces in living systems. Membrane tension in particular is expected to play a central role in many biological processes, but reliable fluorescent probes to image this force in cells do not exist.
We thus realized: Wow, this is a central current challenge in biology that calls for exceptionally innovative solutions from chemistry. Then we thought: Couldn’t we do it like the lobsters do? The lobster pigment, astaxanthin, is a carotenoid with the characteristic orange-red color when cooked (see Figure 1). In living lobster, the twisted chromophore is planarized and polarized until it literally turns blue, or brownish. But the dramatic change of color upon cooking does not originate from molecular transformations but just pure supramolecular mechanochemistry. A similar combination of polarization and planarization in the ground state accounts for the chemistry of vision, particularly color vision, but with fluorescent probes, this demanding combination had never been explored. The first Lobster probe, created by Andrea Fin in the Matile group, had been published in 2012 in Angew. Chem. [1]. It is a tetrathiophene with a methoxy donor at one and a Knoevenagel acceptor at the other terminus (in blue) and three methyls to twist the scaffold out of planarity (in red, Figure 1). In lipid bilayers, a small but detectable red shift of the excitation maximum with increasing membrane order was considered as sufficient for proof of concept.
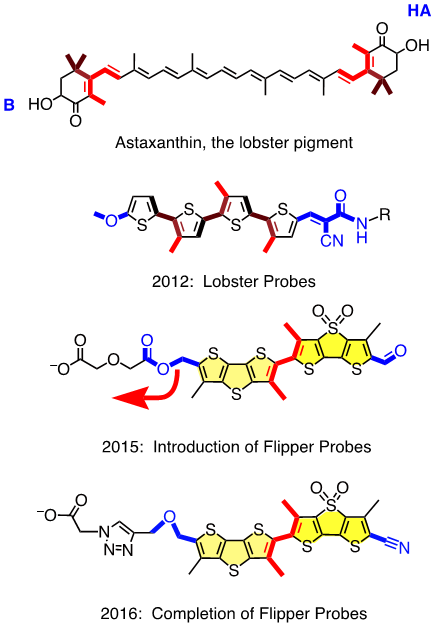
This first “Lobster probe” was particularly ill received, within and beyond the NCCR Chemical Biology. JACS rejected the manuscript with referees laughing out loud, and also one Angewandte referee found it all looking cute but totally useless and without any perspectives. This devastating reception was understandable because the red shifts, that is the mechanosensitivity, were near detection limit, and fluorescence intensity was very weak. To solve these problems, Marta Dal Molin, in a by now legendary Matile group meeting, showed, to everyone’s surprise, a scuba diver with huge shiny flippers. Marta was not thinking of giving up and dreaming of beach vacations. On the contrary, she was thinking about fluorophores with increased surface area to better feel the environment, i.e., with increased mechanosensitivity, and with strong fluorescence also as monomers to keep on shining when twisted out of conjugation. The simplest choice was to bridge two thiophenes in the original probe with either a sulfide donor or a sulfoxide acceptor. This choice was supported by Giovanna Barbarella, who started her career in the late 60s in Geneva and was visiting just in time to tell us, in a most memorable NCCR lecture, all she learned about dithienothiophenes (DTTs) during her life in Bologna. Marta’s flipper probes were accepted for publication in JACS in early 2015 [2] (Figure 1). They worked beautifully in lipid bilayer membranes, bright fluorescence, large red shifts in excitation with increasing membrane order, free partitioning, without disturbing membrane order. Marta’s flippers were clearly better received, even spotlighted in JACS [3], but Cassandra was soon back on stage when they showed the worst possible behavior in cells – no localization, no response to forces, high toxicity. In a few words, a complete disaster.
The problem with Marta’s flippers was easier identified than solved. It was Saeideh Soleimanpour from the Matile group who discovered the headgroup triad we needed: an essential ether as unorthodox donor for the push-pull probe, followed by an essential triazole to prevent the headgroup elimination that hampered Marta’s flipper, and an essential carboxylate to suppress background fluorescence and to deliver and orient the probe in the membrane (Figure 1). Saeideh’s completion of the flipper story was published in late 2016 in a special ChemComm issue on Bioimaging co-edited by Carsten Schultz, a member of the evaluation panel of the NCCR Chemical Biology [4].
Today, Saeideh’s flipper generates increasing excitement in the NCCR community and beyond. Compelling evidence that we have indeed discovered the first fluorescent probe that can faithfully image membrane tension in living cells – strictly speaking the consequences of the applied force as Newton already noted that forces as such cannot be seen – will appear sooner or later. Adai Colom from the Roux group has calibrated the probe in many cells using FLIM (fluorescence lifetime imaging microscopy) and he has found that the observed consequences of membrane tension lead to new insights that are likely to change our understanding in biology. Margot Riggi from the Loewith group has already used Saeideh’s flippers to unravel the role of membrane tension in signal transduction. Scrutinized from all angles within the NCCR community and beyond, flipper probes have, so far, passed all tests with flying colors. Frederick Neumann from the Zumbuehl group has probed flippers by applying pressure to monolayers at the air-water interface [5]. Giuseppe Licari from the Vauthey group tested membrane mechanics by second harmonic generation at liquid-liquid interfaces [6], and Maria Tsemperouli from the Sugihara group will report soon on conductance measurements in black lipid membranes. Moreover, computational simulations are ongoing in the groups of Vauthey and Mareda, an artistic masterpiece from Jiri Mareda is shown in Figure 2.
The completion of the first operational flipper probe to image membrane tension does not mean that we are done. Quite the contrary, there are very good reasons to believe that the best is yet to come – stay tuned!
References
- Fin, A.; Vargas Jentzsch, A.; Sakai, N.; Matile, S., Oligothiophene Amphiphiles as Planarizable and Polarizable Fluorescent Membrane Probes, Chem. Int. Ed., 2012.
- Dal Molin, M.; Verolet, Q.; Colom, A.; Letrun, R.; Derivery, E.; Gonzalez-Gaitan, M.; Vauthey, E.; Roux, A.; Sakai, N.; Matile, S., Fluorescent Flippers for Mechanosensitive Membrane Probes, Am. Chem. Soc., 2015.
- Herman, C., Fluorescent Probes ‘Flip Out’ in Cell Membranes, Am. Chem. Soc., 2015.
- Soleimanpour, S.; Colom, A.; Derivery, E.; Gonzalez-Gaitan, M.; Roux, A.; Sakai, N.; Matile, S., Headgroup Engineering in Mechanosensitive Membrane Probes, Commun. 2016.
- Neuhaus, F.; Zobi, F.; Brezesinski, G.; Dal Molin, M.; Matile, S.; Zumbuehl, A., Correlation of Surface Pressure and Color Hue of Planarizable Push-Pull Chromophores at the Air/Water Interface, Beilstein J. Org. Chem. 2017.
- Licari, G.; Beckwith, S.; Soleimanpour, S.; Matile, S.; Vauthey, E., Detecting Order and Lateral Pressure at Biomimetic Interfaces Using a Mechanosensitive Second-Harmonic-Generation Probe, Phys. Chem. Chem. Phys., 2018.

Leave a comment
The editors reserve the right not to publish comments or to abridge them.